Recent
Advances in Human Neurophysiology.
March 1998. Okazaki, Japan.
Comparative Analysis using VEPs showed many abnormalities
in autistic respect to healthy children.
Recent Advances in Human Neurophysiology.
March 1998. Okazaki, Japan.
AGUILAR
L. C., MARTIN R., CRUZ S., ROSIQUE P., ISLAS A.AND
ALFAROF.
Instituto de Investigaciones en Neuroplasticidad
y Desarrollo Celular. Departamento de Biologia
Celular y Molecular de la Universidad de Guadalajara.
Guadalajara, Jalisco MEXICO.
Contents
• Introduction
• Methods
• Results
• Discussion and
Conclusion
• References
INTRODUCTION
The knowledge
about the neural sources of some components of
the flash VEPs and the normal sequence of these
components is limited. Anatomical and physiological
studies has shown visual projections to areas
outside the occipital lobe (Von Essen 1979, Kuypers
et al. 1965, Bignall and Imbert 1969, Boyd et
al. 1971) indicating cortico-cortical connections
from visual association areas to parietal lobe,
premotor, prefrontal cortex and to middle and
inferior temporal gyri. Recordings in humans (Walter
and Walter 1949; Brazier 1964) have shown VEPs
in frontal and temporal regions. Studies performed
by Hammond et al. 1989, showed that the topographic
flash VEPs can provide data on the location of
lesions remote from the occipital area, where
usually the components were asymmetrical, caused
by a depression or enhancement of some component.
In other hand the flash VEPs are in many cases
of children with mental retardation and autism,
the only possibility to perform a visual evoked
potential study, for there is not patient collaboration
for performing the study which has to be performed
in sleep stage. We performed a prospective topographic
flash VEPs study in normal and autistic children
in order to know the normal sequence of components,
the grade of interhemispheric symmetry for each
area and the comparison with autistic children.
METHODS
Topographic flash
VEPs were recorded from a control group (n=40)
and from autistic patients (DSM IV, n=103), both
sexes, between 3 and 13 years. The stimulation
was performed using a white stroboscopic xenon
flash (Grass PS22). All stimuli were presented
binocularly with a variable repetition rate during
sleep (stage II), 16 electrodes were placed according
to 10-20 international system, amplifier bandpass
filters 1-35 Hz, 200 epochs were averaged and
obtained twice. Symmetry analysis of each area
was performed using Pearson Coefficient (PC) to
know the linear correlation and energy ratio (ER)
to know the symmetry in area below the curve,
for 50-200 and 200-400 milliseconds (ms) segments,
the result of each area on a specific segment
of time of every autistic child was compared with
a control group using Z transform (p<0.025)
for knowing the significant deviations.
RESULTS
The following
sequence of negative components was observed in
normal controls: The initial response was detected
in frontopolar regions (FP1-FP2), followed by
occipitals (O1-O2), continuing with parietals
(P3-P4), then temporals (T5-T6), followed by frontopolars,
frontals (F3-F4), centrals (C3-C4), followed by
occipitals, parietals and temporals (Fig.1 and
2). The linear correlation analysis showed high
symmetry with a values higher to 0.70 (Pearson
coefficient) for p<0.025. The symmetry analysis
of ABC studied through ER showed in control group
values of one side respect to contra lateral no
higher than 2.5 times and no lower than 0.4 times.,
this for a P<0.025 (Fig. 1 and 2), only the
frontopolars (Fp1-Fp2) in the 50-200 ms segment,
the frontolaterals (F7-F8) and anterior temporals
(T3-T4) showed low symmetry in this parameters,
observing a grate dispersion in the values of
these regions.
|
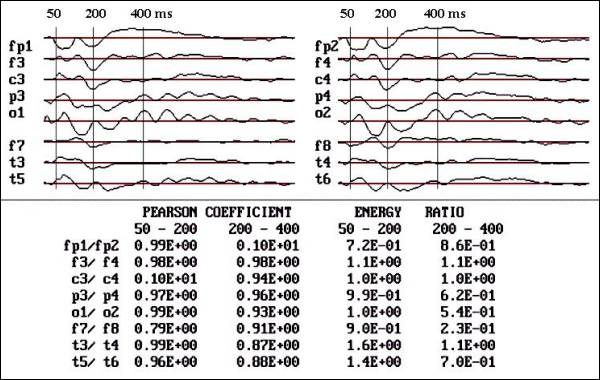
Fig.
1.- Flash VEPs of a control child, female
12 years old. Notice the sequence of
negative components and the high symmetry
in linear correlation and area below
the curve studied by energy ratio
|
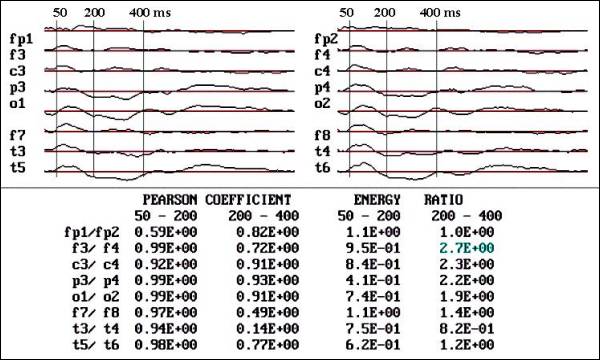
Fig. 2.- Flash VEPs
of a 6 month old male. Observe the
high symmetry quantified by Pearson
coefficient (PC) (Linear correlation)
and energy ratio (ER), only the T3-T4
and F7-F8 and FP1-FP2 in 50-200 ms
segment showed low PC and ER.
|
In autistic children
the sequence of components showed many abnormalities
including depression, enhanced of components,
like to previous observations in patients with
brain damage reported by Hammond et al 1989 (Fig.
3 and 4), or premature apparition of a specific
component like the negative component (NC) in
parietal (Fig. 4) that usually appears after the
occipital N100 (Fig. 1 and 2) in controls, this
abnormalities were observed mainly in right posterior
regions ; the reduction of voltage (mainly in
P4,T6 and O2) was mostly seen (Fig. 3). The symmetry
analysis shows many deficiencies in linear correlation
where parietals (P3-P4) presented significant
low PC (p<0.025) in 63% of patients and 66
% in T5-T6 for the 200-400 ms segment.
Also the autistic
group showed significant asymmetries of ER (p<0.025)
in 56% of the patients in P3-P4, 40 % in O1-O2
and 39 % in T5-T6 for the 200-400 ms segment.
| 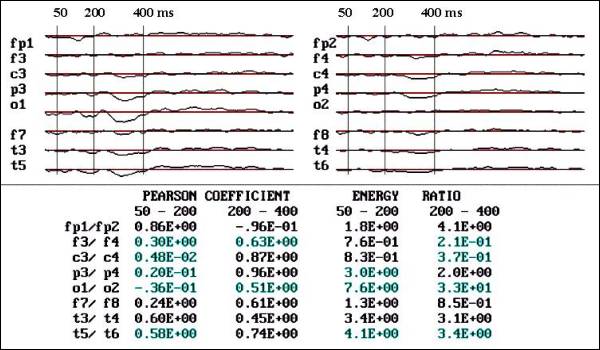
Fig.
3.- Flash VEPs of autistic child, 6
year old male. Notice the great reduction
of voltage in 02, T6 and P4, and the
very low Pearson coefficient in the
mentioned areas |
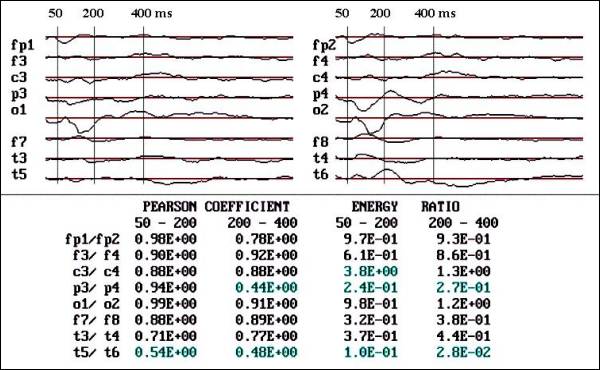
Fig. 4.- Observe
the premature response of negative
component in P4, which appear prior
to N100 in O2, also the Pearson coefficient
and energy ratio are very low in P3-P4
and T5-T6.
|
DISCUSSION
AND CONCLUSIONS
The mentioned
results indicate that there is a normal sequence
of responses after a flash stimulation that can
be reflecting the cognitive process. The symmetry
analysis in the control healthy group showed that
the high symmetry observed in frontal, central,
parietal, occipital and temporal posterior regions
was no age-dependent, this symmetry was high including
in a 6 month old normal child (Fig. 2). The study
of mentioned sequence in autistic children and
the symmetry analysis through PC and ER that contributed
greatly to perform a more objective and reliable
study, indicated that parietal and temporal regions
are frequently affected in autism, only 2 autistic
children of 103 did not show some abnormality
of Flash VEPs in this areas, but one of them showed
in the EEG spikes in both temporal lobes (T3 and
T4) and the second one showed spikes in frontal
regions and seizures every week. These results
strongly suggest that the parietal and temporal
regions are affected in autistic children and
according to Hammond et al. 1989, these asymmetries
can be reflecting lesion or damage.
The neuropsychological
analysis performed in the autistic group showed
that the language and visual motor skills were
the most deficient functions. This functions are
related with temporal (Mc Carthy and Warrington
1990) and parietal ( Perenin and Vighetto, 1988)
regions. We conclude that parietal and temporal
regions are high frequently affected in autism.
REFERENCES
Von Essen DC. 1979.
Visual areas of the mammalian cerebral cortex.
Annu Rev Neurosci. 2:227-263.
Kuypers HGJM. Szwarcbart
MK. Mishkin M. Rosvold HE. 1965. Occipitotemporal
corticocortical connections in the rhesus monkey.
Exp Neurol. 11: 245-262.
Bignall KE. Imbert
M. 1969. Polisensory and corticocortical projections
to frontal lobe of squirrel and rhesus monkeys.
Electroencephallogr Clin Neurophysiol 26: 206-215.
Boyd EH. Pandya
DN. Bignall KE. 1971. Homotopic and nonhomotopic
interhemispheric cortical projections in the squirrel
monkey. Exp Neurol. 32: 256-274
Walter VJ and Walter
WG. 1949. The central effects of rythmic sensory
stimulation. Electroencephalogr Clin Neurophysiol.
1: 57-86.
Brazier MAB. 1964.
Evoked responses recorded from the depths of human
brain. Ann NY Acad Sci. 112: 33-59.
Hammond EJ. Barber
CP. Wilder BJ. 1989. Flash Visual Evoked Potential
Topographic Mapping: Normative and Clinical Data.
In: Topographic Brain Mapping of EEG and Evoked
Potentials. (Maurer K Ed) Springer-Verlag.
McCarthy R. Warrintong
EK. 1990. Cognitive Neuropsychology. A clinical
Introduction. Academic Press.
Perenin MT. Vighetto
A. 1988. Optic Ataxia a specific disruption in
visomotor mechanism. Brain. 111: 643-674.

The Fibroblast Growth Factor 2 (FGF2) Improves
VEPs in Autistic Children.
Recent Advances in Human Neurophysiology.
March 1998. Okazaki, Japan.
AGUILAR L. C., CRUZ S., MARTIN R., ROSIQUE P.,
ALFARO F., ISLAS A., AND CANTU J.M.
Instituto de Investigaciones en Neuroplasticidad
y Desarrollo Celular . Departamento de Biologia
Celular y Molecular de la Universidad de Guadalajara.
CIBO, IMSS. Guadalajara, Jalisco, MEXICO A.P.
3920.
Contents
• Introduction
• Methods
• Results
• Discussion and
Conclusion
• References
INTRODUCTION
FGF2 is a protein
that has shown neurotrophic effect in many areas
of the embryonic (Eckenstein et.al., 1990. Weise
et. al., 1993), foetal (Walicke et. al., 1986.
Morrison et. al., 1986. . Deloulme, et. al., 1991)
and adult brain( Matsuda et al. 1990). Its in
vitro effects include survival, neurite extension
(Peulve P. Et. al., 1994), increase in choline
acetyltransferase (CAT) activity, dopamine levels
(Aguilar et. al. 1994a).
In several animal models of brain damage as trauma
(Mocchetti et. al. 1995, Anderson et. al., 1988),
hypoxia/ischemia (Aguilar et. al., 1994ª, Nakata
et. al., 1993), kainic acid (Rudge et. al., 1995),
and pathway sections (Koshinaga et. al., 1993),
etc.) have shown that the FGF2 is capable of diminishing
the degree of lesion. Studies in which FGF2 was
administered after damage (Anderson et. al., 1988),
showed a significant improvement in morphological,
neurochemical (Otto, D. and Unsicker, K. 1990.)
and neurophysiological (Aguilar et. al. 1994a)
parameters. Previous clinical studies in intellectual
disabilities showed a significant improvement
in mental retardation (Aguilar et. al., 1993)
and language disabilities (Aguilar et. al.,1994b).
Symmetry analysis
of Flash VEPs, in previous studies carried out
by our group, demonstrates to be a reliable and
objective procedure for quantifying abnormalities
in autistic children, when using Pearson Coefficient
(PC) to analyse interhemispheric linear correlation
and Energy Ratio (ER) to compare the voltage generated
in a specific segment of time(area below the curve)
in both hemispheres.
METHODS
With the purpose
of improving the impairment of autistic children,
FGF2 was used, applied subcutaneously at dosage
of 0.2 mg/kg, every two weeks during 12 months.
The autistic group (n=29, both sexes between 3
and 8 years of age), was studied using flash VEPs,
with 16 electrodes placed according to 10-20 international
system. VEPs were analysed in 2 segments, 50-200
and 200-400 ms through PC and ER, PC was expressed
as a value between 1 and –1, the values
lower than 0.7, were considered as significant
deviations (p<0.025) when compared to control
healthy group (CHG) and ER was expressed as a
ratio in which the higher value of one area (
any side) was normalised to 1 and the contra lateral
as a ratio, the values lower 0.4 were significant
deviated (p<0.025) of a CHG indicating asymmetry.
The areas that in the initial study showed significant
deviations (P<0.025) respect to normal group
were totalled and compared before vs. after 12
month of treatment, trough t-test.
RESULTS
The symmetry of
area below the curve or ER in P3-P4 and O1-O2
showed significant increase (p<0.004) in segment
50-200 ms (Fig. 1) and in 200-400 ms segment,
P3-P4 and T5-T6 (P<0.004) increase after 12
months of treatment (Fig. 1). P3-P4 and T5-T6
showed in the initial study the grater number
of asymmetries in PC mainly in 200-400 ms segment
and after FGF2 treatment a significant reduction
(p<0.01) was observed in all areas in both
segments (Fig. 2).
| 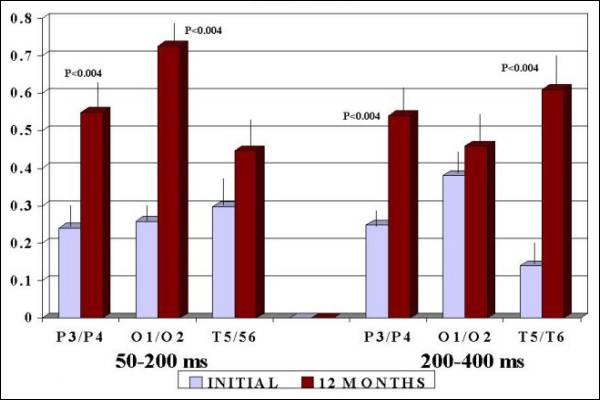
Fig.
1. Energy Radio (ER) before and after
FGF2 Therapy in Autistic Children. Observe
the significant increase
|
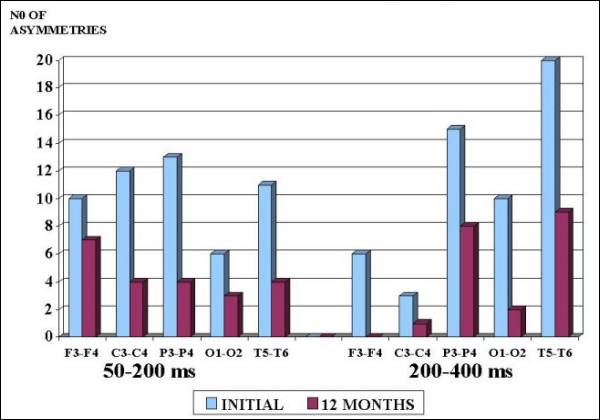
Fig. 2. Deviations
in Pearson Coefficient (PC) before
and after 12 months of FGF2 Therapy
in Autistic Children. Observe the
significant reduction (P<0.01)
|
DISCUSSION
AND CONCLUSIONS
The parietals
(P3-P4) improved significantly in ER and less
in PC (p<0.08), this probably due to the fact,
that first the number of responding neurones increase
and then the functional organisation, in general
the improvement was higher in ER that in PC. T5-T6
increase significantly in 200-400 ms in both symmetry
analysis (ER and PC) indicating improvement in
the late components of this regions, probably
related to the fact that the best response in
neuropsychological evaluation was in language,
function highly related with temporal regions
(Head, H. 1926).
TWe conclude that FGF2 improves flash VEPs mainly
in parietal, temporal and occipital regions of
autistic children after 12 months of treatment
(Fig 3 a, 3 b, 4 a and 4 b), this correlated with
improving in language, visual motor maturation
and social behaviour, areas that showed the best
evolution in the neuropsychological evaluation.
The most noticeable improvement was the recovery
of the area below the curve in many patients in
which the initial study showed absence or very
low response in parietal, temporal and occipital
regions mainly in the right side (Fig. 3 a and
3 b).
| 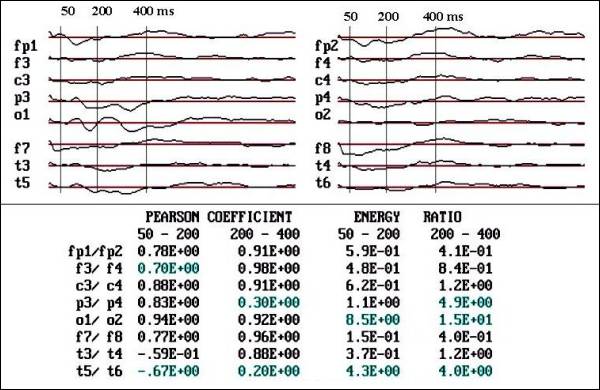
Fig.
3 a. Notice the absence of response
in O2 and reduction of voltage in T6
and P4, respect to contra lateral regions
in autistic child ( male, 4 years old). |
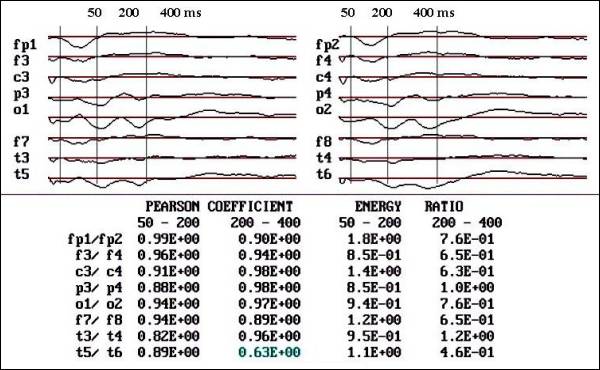
Fig. 3 b. Notice
the recovery in voltage and area below
the curve in O2, T6 and P4, also observe
that the Pearson coefficient increase
in the same autistic patient after
12 months of FGF2 therapy.
|
|
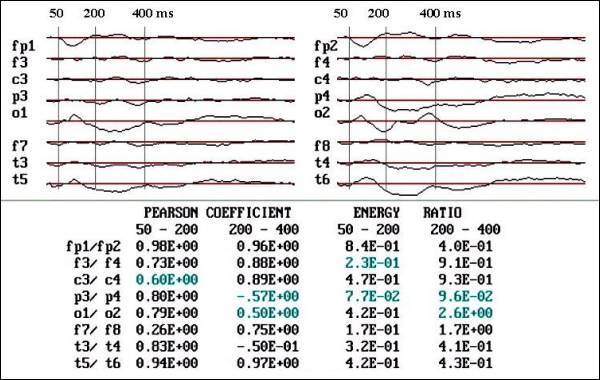
Fig.
4 a. Observe the low Pearson coefficient
and the asymmetries in area below the
curve in P3-P4, O1-O2 and T5-T6.
|
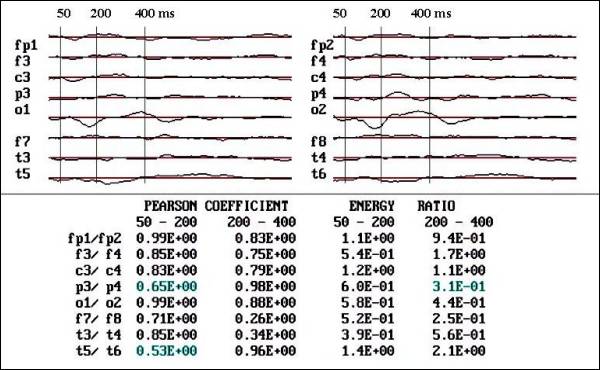
Fig. 4 b. Notice
the increase in Pearson coefficient
and reduction of asymmetries in voltage
(energy ratio) in P3-P4, O1-O2 and
T5-T6 after 12 months of FGF2 treatment.
|
REFERENCES
Aguilar_LC; Islas_A;
Rosique_P; Hernandez_B; Portillo_E; Herrera_JM;
Cortes_R; Cruz_S; Alfaro_F; Martin_R; et_al Psychometric
analysis in children with mental retardation due
to perinatal hypoxia treated with fibroblast growth
factor (FGF) and showing improvement in mental
development. J Intellect Disabil Res, 1993 Dec,
37 ( Pt 6):, 507-20
Aguilar, L.C.,
Islas, A., Morales, A., Alfaro, F., Cruz, S.,
Martin, R., and Cantu, J.M. 1994a. Estudios preclinicos
de la administración del Factor de Crecimiento
Fibroblástico en daño cerebral. Capitulo VI. 83-94.
En Avances en la Restauración del Sistema Nervioso.
Aguilar-Rebolledo, F. Ed. Vicova Editores.
Aguilar, L.C.,
Rosique, P., Cruz, S., Martin, M., Alfaro, F.,
Islas, A., and Cantu, J.M. 1994b. Administración
del factor Fibroblástico de Crecimiento en disfunciones
neurológicas consecutivas a hipóxia-isquemia perinatal.
Capitulo VII. 95-106. En Avances en la Restauración
del Sistema Nervioso. Aguilar-Rebolledo, F. Ed.
Vicova Editores.
Anderson, K.J.,
Dam, D., Lee, S. and Cotman, C.W. 1988. Basic
fibroblast growth factor prevents death of lesioned
cholinergic neurons in vivo. Nature. 332:360-361.
Deloulme_JC; Baudier_J;
Sensenbrenner_M Establishment of pure neuronal
cultures from fetal rat spinal cord and proliferation
of the neuronal precursor cells in the presence
of fibroblast growth factor. J Neurosci Res, 1991
Aug, 29:4, 499-509.
Eckenstein_FP;
Esch_F; Holbert_T; Blacher_RW; Nishi_R Purification
and characterisation of a trophic factor for embryonic
peripheral neurons: comparison with fibroblast
growth factors.
Neuron, 1990 Apr, 4:4, 623-31.
Head, H. (1926).
Aphasia and kindred disorders of speech. Cambridge;
Cambridge University Press.
Koshinaga_M; Sanon_HR;
Whittemore_SR Altered acidic and basic fibroblast
growth factor expression following spinal cord
injury. Exp Neurol, 1993 Mar, 120:1, 32-48
Matsuda, S., Saito,
H. and Nishiyama, N. 1990. Effect of basic growth
factor on neurons cultured from various regions
of postnatal rat brain. Brain Res. 520: 310-316.
Mocchetti_I; Wrathall_JR
Neurotrophic factors in central nervous system
trauma. J Neurotrauma, 1995 Oct, 12:5, 853-70
Morrison, R.S.,
Sharma A., de Vellis, J. and Bradshaw
R.A. 1986. Basic
fibroblast growth factor supports the survival
of cerebral cortical neurons in primary culture.
Proc. Natl. Acad. Sci. USA. 83:75377541.
Nakata_N; Kato_H;
Kogure_K Protective effects of basic fibroblast
growth factor against hippocampal neuronal damage
following cerebral ischemia in the gerbil. Brain
Res, 1993 Mar 12, 605:2, 354-6
Otto, D. and Unsicker,
K. 1990. Basic FGF reverses chemical and morphological
deficits in the nigrostriatal system of MPTP-treated
mice. J. Neurosci. 10:1912-1921.
Peulve_P; Laquerriere_A;
Hemet_J; Tadie_M Comparative effect of alpha-MSH
and b-FGF on neurite extension of fetal rat spinal
cord neurons in culture. Brain Res, 1994 Aug 22,
654:2, 319-23
Rudge_JS; Pasnikowski_EM;
Holst_P; Lindsay_RM Changes in neurotrophic factor
expression and receptor activation following exposure
of hippocampal neuron/astrocyte cocultures to
kainic acid. J Neurosci, 1995 Oct, 15:10, 6856-67
Walicke, P., Cowan,
W.M., Ueno, N., Baird, A. and Guillemin R. 1986.
Fibroblast growth factor promotes survival of
dissociated hippocampal neurons and enhances neurite
extension. Proc. Natl. Acad. Sci. USA. 83:3012-3016.
Walicke, P.A. 1988.
Basic and acidic fibroblast growth factors have
trophic effects on neurons from multiple CNS regions.
J. Neurosci. 8:2618-2627.
Weise_B; Janet_T; Grothe_C Localization
of bFGF and FGF-receptor in the developing nervous
system of the embryonic and newborn rat. J Neurosci
Res, 1993 Mar 1, 34:4, 442-53

The FGF2 Improves
VEPs in Dyslexic Children (DCh).
Recent Advances in Human Neurophysiology.
March 1998. Okazaki, Japan.
L. C.
AGUILAR, P. ROSIQUE, F. ALFARO, R. MARTIN, S.
CRUZ, A. ISLAS, AND J. M. CANTU.
IINEDEC, a.p. 3920, Guadalajara, Jal, Mexico.
IFC. CUCBA. U.de G. Div. de Genetica, CIBO.IMSS.
Contents
• Introduction
• Results
• Discussion and
Conclusion
• References
INTRODUCTION
The FGF 2, is
a neurotrophic factor, with effect on CNS neurons
(cortex, hippocampus (HP), striatum (ST), thalamus,
etc.( Eckenstein et. al., 1990, Weise et. al.,
1993, Walike et. al., 1986, Deloulme, et. al.,
1991) showing in vitro increase of survival and
neurite outgrowth (Walicke, 1988); in vivo increases
CAT activity in HP and ST and dopamine levels
in ST (Aguilar et.al., 1994a). FGF 2 reverses
morphological deficits in dopaminergic neurons
after MPTP (Otto and Unsicker, et.al. 1990), and
ameliorates learning deficits in basal-forebrain
lesioned mice (Ishihara, et.al., 1992). In mentally
retarded patients FGF 2 improves mental development
(Aguilar et al., 1993). Also improves language
disabilities and visomotor deficits in children
with these pathologies (Aguilar, et.al. 1994b).
We report here, the effect of FGF 2 therapy in
children with dyslexia in whom phonological, semantic
and visual-spatial deficits were observed. 41
patients of both sexes, in ages between 8 and
12 years of age, separated in two groups were
examined. Topographic flash VEPs were recorded
from a treated group (TG) n=26 and from untreated
group (n= 15), both sexes, between 8 and 12 years.
The stimulation was performed using a white stroboscopic
xenon flash (Grass PS22). All stimuli were presented
binocularly with a variable repetition rate during
awake state, 16 electrodes were placed according
to 10-20 international system, amplifier bandpass
was 1-35 Hz, 200 epochs were averaged and obtained
twice. Symmetry analysis of each area was performed
using Pearson coefficient (PC) to know the linear
correlation and energy ratio (ER) to know the
symmetry in energy (comparison of the area below
the curve of one region respect to contra lateral),
for 50-200 and 200-400 milliseconds (ms) segments,
the result of each area in a specific segment
of time of every dyslexic child was compared with
a control group (n=25, age matched) using Z transform
(p<0.025) in order to know the significant
deviations.
FGF2 was administered at dosage
of 0.2 mg/kg of body weight every 2 weeks during
12 months to TG, the UG received only neurophsycological
therapy.
RESULTS
The VEPs showed
in the initial study that the PC was significantly
(p<0.025) reduced in 58 % of dyslexic respect
to normal controls in at least one segment and
the 48 % in the 200-400 ms segment. In ER 48%
of patients showed asymmetries at least in one
segment, a great reduction of voltage and area
of N100 component on left occipital and temporal
regions was observed in 28 % of dyslexic children
(Fig. 1 a and 2 a ), causing the mentioned asymmetries
in ER and PC, in occipital regions only the 26
% showed asymmetries.
After 12 months of treatment with FGF2, a significant
(p<0.01) improvement in PC was observed in
T5-T6 (Fig. 3) in 200-400 ms segment, the ER improved
significantly (p<0.01) in both segments of
T5-T6 (Fig. 3 ),. The UG did not show significant
changes.
|
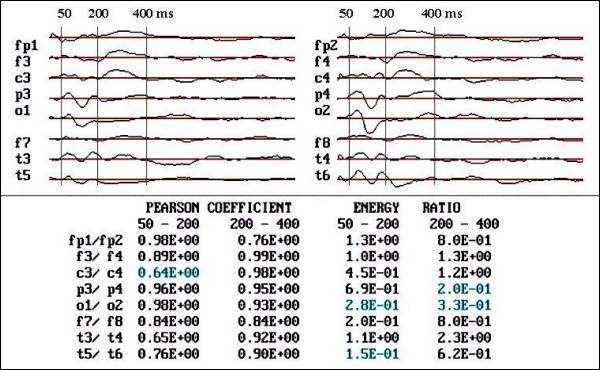
Fig.
1 a.- Case one, before treatment. Observe
the reduction in voltage and area below
the curve in O1 and T5 respect to contralaterals,
in dyslexic child (male, 7 years old).
|
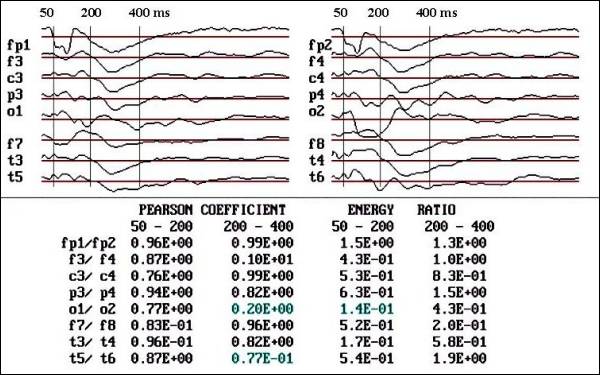
Fig. 2 a.- Case two,
before treatment. Observe the reduction
of area below the curve in O1 and T5
respect to contralaterals in dyslexic
child (male 9 years old).
|
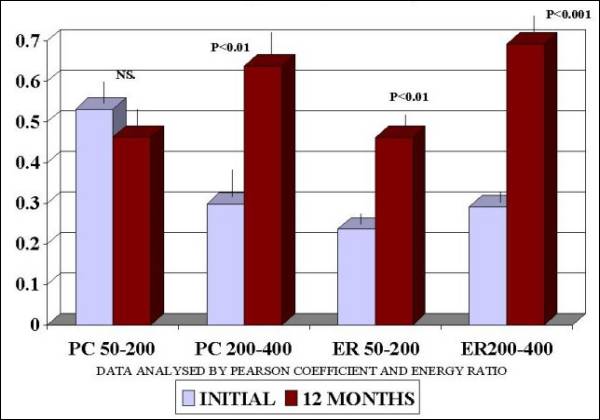
Fig.
3.- Notice the significant increase in
Pearson coefficient (p<0.01) in 200-400
ms. and energy ratio in 50-200 and 200-400
ms segments (p<0.01) of T5-T6 after
12 months of FGF2 therapy |
DISCUSSION
AND CONCLUSIONS
The improvement
in ER was associated with the increase of voltage
(area below the curve) on the regions in which
previously was reduced ( Fig. 1 b and 2 b), this
occurs more frequently on left occipital and temporal
regions. This correlates with neurophsycological
studies in which several authors demonstrate anatomical
deficits on these regions of dyslexic children
(Greenblat, 1973, Ajax et al.1977, Staller et
al. 1978).. The above mentioned suggests an increase
on the number of the responding neurons of these
regions, probably due to new pathways formation
between geniculate lateral nucleus and area IV
of occipital cortex as well as between occipital
and temporal cortex, there was also an increase
in the activity of neurons of the mentioned areas.
The VEPs improvement in PC suggest a better synchrony
and organisation.
We conclude that the flash VEPs are useful for
detecting the neurophysiological abnormalities
in dyslexic patients and for monitoring the effects
of treatments like the one with FGF2, in which
a significant improvement of dyslexic children
was observed in neuropsychological and neurophysiological
parameters.
|
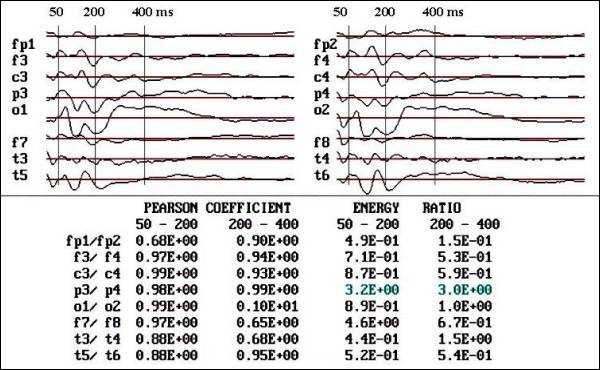
Fig.
1 b.- Case one, after 12 months treatment.
Notice the recovery in area below the
curve, Pearson coefficient and energy
ratio in O1 and T5. |
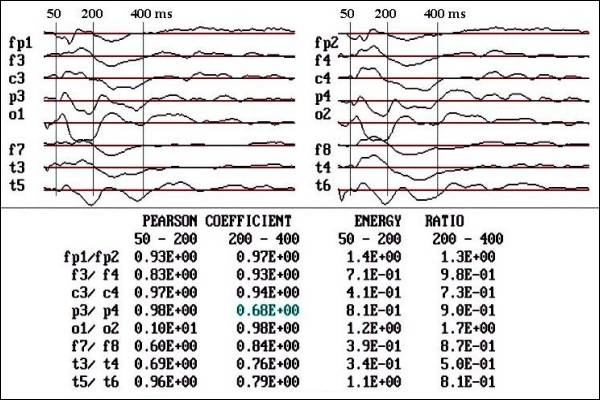
Fig. 2 b.- Case two,
after treatment. Observe the improvement
in energy ratio of T5-T6 and O1-O2 in
50-200 ms. segment.
|
REFERENCES
Aguilar_LC; Islas_A;
Rosique_P; Hernandez_B; Portillo_E; Herrera_JM;
Cortes_R; Cruz_S; Alfaro_F; Martin_R; et_al Psychometric
analysis in children with mental retardation due
to perinatal hypoxia treated with fibroblast growth
factor (FGF) and showing improvement in mental
development. J Intellect Disabil Res, 1993 Dec,
37 ( Pt 6):, 507-20
Aguilar, L.C., Islas, A., Morales,
A., Alfaro, F., Cruz, S., Martin, R., and Cantu,
J.M. 1994a. Estudios preclinicos de la administración
del Factor de Crecimiento Fibroblástico en daño
cerebral. Capitulo VI. 83-94. En Avances en la
Restauración del Sistema Nervioso. Aguilar-Rebolledo,
F. Ed. Vicova Editores.
Aguilar, L.C., Rosique, P.,
Cruz, S., Martin, M., Alfaro, F., Islas, A., and
Cantu, J.M. 1994b. Administración del factor Fibroblástico
de Crecimiento en disfunciones neurológicas consecutivas
a hipóxia-isquemia perinatal. Capitulo VII. 95-106.
En Avances en la Restauración del Sistema Nervioso.
Aguilar-Rebolledo, F. Ed. Vicova Editores.
Ajax, E.T., Schenkenberg, T.,
& Kasteljanetz, M. (1997). Alexia without
agraphia. Archives of Neurology (Chicago), 17,
645-652.
Deloulme_JC; Baudier_J; Sensenbrenner_M
Establishment of pure neuronal cultures from fetal
rat spinal cord and proliferation of the neuronal
precursor cells in the presence of fibroblast
growth factor. J Neurosci Res, 1991 Aug, 29:4,
499-509.
Eckenstein_FP; Esch_F; Holbert_T;
Blacher_RW; Nishi_R Purification and characterisation
of a trophic factor for embryonic peripheral neurons:
comparison with fibroblast growth factors.
Neuron, 1990 Apr, 4:4, 623-31 .
Greenblatt, S.H. (1973). Alexia
without agraphia or hemianopia; anatomical analysis
of autopsied case, Brain, 96, 307-316.
Ishihara, A., Saito, H., and
Nishiyama, N. 1992. Basic Fibroblast Growth Factor
ameliorates learning deficits in basal forebrain
lesioned mice. Jpn. J. Pharmacol. 59: 7-13.
Otto, D. and Unsicker, K. 1990.
Basic FGF reverses chemical and morphological
deficits in the nigrostriatal system of MPTP-treated
mice. J. Neurosci. 10:1912-1921.
Staller, J., Buchanan, D., Singer,
M., Lappin, J., & webb, W. (1978), Alexia
without agraphia: An experimental case study,
Brain and Language, 5, 378-387.
Walicke, P., Cowan, W.M., Ueno,
N., Baird, A. and Guillemin R. 1986. Fibroblast
growth factor promotes survival of dissociated
hippocampal neurons and enhances neurite extension.
Proc. Natl. Acad. Sci. USA. 83:3012-3016.
Walicke, P.A. 1988. Basic and
acidic fibroblast growth factors have trophic
effects on neurons from multiple CNS regions.
J. Neurosci. 8:2618-2627.
Weise_B; Janet_T; Grothe_C Localization
of bFGF and FGF-receptor in the developing nervous
system of the embryonic and newborn rat. J Neurosci
Res, 1993 Mar 1, 34:4, 442-53